Abstract 
Results pertaining to the germination percentage of pre-soaked seeds in a series of temperature regimes viz., 100C, 150C, 200C and 250C have revealed significant increase among seed sources in each of the three conifer species of Garhwal Himalaya. Soaking of the seeds for 24 hours in GA3 solution had shown maximum germination in A. pindrow (45.0±4.19%), C. torulosa (57.0±3.40%) and P. smithiana (56±6.01%) as compared to untreated (control) seeds. It has also been observed that GA3 treatment caused an appreciable shortening of the germination period by 10 days. Therefore, seeds of these commercially important tree species should be pre-treated particularly with GA3 for 24 hours for getting enhanced germination. It is important to point out here that the seeds of each of the three species reflect poor germination in nature due to snow cover, seed decay, prevalence of excess water and lack of maintenance, however, because of increasing demand for large quantities of tree seeds for reforestation programmes, pre-sowing treatments are useful to improve the rate and percentage of germination.
Key words:Germination percentage, Gibberellic acid, Conifers species, Himalaya, Germination value
Introduction 
Genetic variation is manifested through provenance tests, designed to assess the degree and pattern of variation across species ranges. The typical experimental approach consists of collecting open-pollinated seeds in a single season, from trees within portions of the range and evaluating the performance of the resulting seedlings in formal "common garden test" at several locations within the range. Frequently as a first investigative step, the germination characteristics of these seeds are evaluated under conditions, which allow comparison of geographical provenances, or families within provenance. Provenance differences are sometimes assumed to have a genetic basis. However, such tests are actually based on the phenotypic variations among seed lots from provenances, and not on genetic variation. Rowe (1964), and Baskin & Baskin (1973) have noted, that the difference in seed characteristics of ecologically important provenances may also be due to genetic variability.
The primary consideration in bringing out genetic improvement in a particular species is the development of a sound scientific breeding programme, based upon the available genetic variability. However, progress in improvement in many species has been poor due to failure in utilising the existing variability in tree improvement. The success of tree breeding programmes relies mostly on the ability to identify and deploy superior trees. Decisions on how, when, and what to select, are made (or should be made) taking into account genetic and economic information. Selection in tree breeding programmes, therefore should be based upon genetic information generated from progeny tests. Plant growth substances are involved in seed germination and subsequent growth; their specific roles are still obscure. In order to have a better understanding of the functions of plant growth, subsequent during these processes, it is important to identify and quantify them through imbibitions, from growth of the seedlings. Gibberellic acid (GA3) has been shown to promote germination of seed (Vogt, (1970); Krishnamurthy, (1973); Chandra & Chauhan, (1976)) however, the germination percent increased in the seeds of Nothofagus obliqua, when they were pre-chilled after soaking in GA3 Solution for 24 hours (Shafiq, 1980). Singh (1973) reported that spruce seeds germinated comparatively more profusely than silver fir, and further recorded that enough seeds become available for raising sufficient planting stock. Keeping this in view the present study was aimed at understanding the role of GA3 treatment for seed germination in different provenances of Abies pindrow, Cupressus torulosa and Picea smithiana.
Materials and Methods 
The present study was undertaken in five provenances each of three important coniferous species (15 provenances) i.e., Abies pindrow, Cupressus torulosa and Picea smithiana from three districts viz. , Pauri, Tehri and Chamoli of Garhwal Himalaya. The details of the study areas have been presented in Table-1.
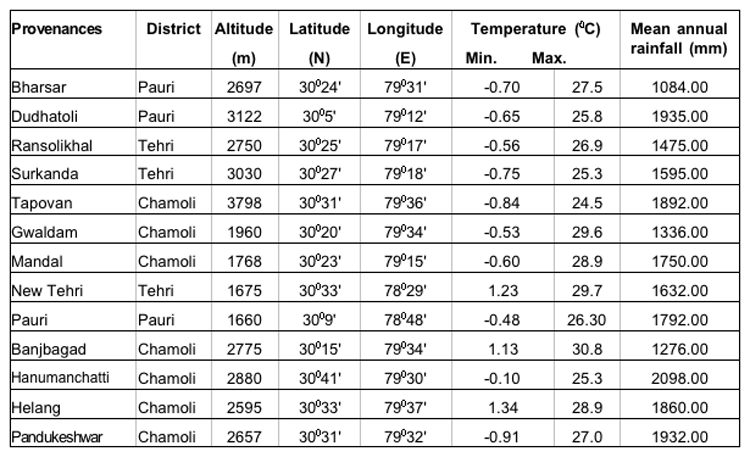
Table 1: Geographical and meteorological descriptions of the seed sources of A. pindrow, C. torulosa and P. smithiana.
The studies pertaining to provenance variation and seed germination after pre-soaking treatment were carried out under laboratory conditions at various temperatures viz. , 100C, 150C, 200C and 250C, inside a seed germinator (Model No. 8LT-SGL CALTAN). The seeds of all the provenances of each species were germinated at similar temperatures after applying following treatment to each set:
Treatment 1- Soaking of the seeds in distilled water at room temperature (250C) for 24 hours (as control). Treatment 2 -Soaking of the seeds in Gibberellic acid (GA3 100ppm) at room temperature for 24 hour (treatment).
For germination, the seeds in five replicates of 100 seeds each were placed in Petri dishes containing two filter papers, kept in the germinator, and maintained at desired temperature. Observations were recorded daily for germinated /non-germinated seeds up to 21 days. Radical emergence was taken as the criteria for germinability. The germinated seeds after cessation of the experiment were transplanted to the polythene bags in the nursery.
The data on seed germination was recorded and quantified in terms of percent germination and germination value. Percent germination was the value of seeds germinated at the completion of the germination period, whereas, germination value is an index, combining speed and completeness of germination; which according to Czabator (1962) can be expressed as: GV= PV X MDG, where, GV is germination value, PV is the peak value of germination, and MDG is mean daily germination.
Statistical Analysis:
The statistical analysis of each parameter was carried out on mean values and the analysis of variance (ANOVA) was performed using SPSS package. The critical difference (CD) was calculated as: CD = SEd X t0.01, where, SEd is the standard error of differences calculated as SEd = 2Me/2Results and Discussion 
Germination of seeds of various provenances after pre-soaking treatment with GA3 under different temperature regimes, (i.e., 100C, 150C, 200C and 250C) has yielded significant differences in seed germination. The data analysed for its variance, has revealed remarkable variation amongst different seed sources, which have been presented in (Table-5). The detailed treatment-temperature interactions are given below:-
[[saubheading text="Soaking of seeds in distilled water (as control):]] In Abies pindrow the maximum germination of seeds at 100C (32.0 ± 2.00%) and 150C (18.0 ± 4.37%) was recorded in Tapovan provenance, whereas, at 200C (22.0 ± 9.59%) and 250C (19.0 ± 1.0%) in Ransolikhal provenance. On the other hand maximum germination values at 100C, 150C, 200C and 250C was observed for Tapovan (0.67 ± 0.15), Surkanda (0.39 ± 0.09), Ransolikhal (0.49 ± 0.38) and Bharsar (0.30 ± 0.28) provenances respectively. The minimum germination of seeds at these temperatures were recorded in Surkanda (21.0 ± 2.92%), Bharsar (14.0 ± 4.01%), Dudhatoli (15.0 ± 3.54%) and Tapovan (10.0 ± 2.74%) provenances and minimum germination values for Dudhatoli (0.26 ± 0.05), Ransolikhal (0.22 ± 0.06), Tapovan (0.11 ± 0.01) and Surkanda (0.09 ± 0.03) provenances respectively (Table-2).
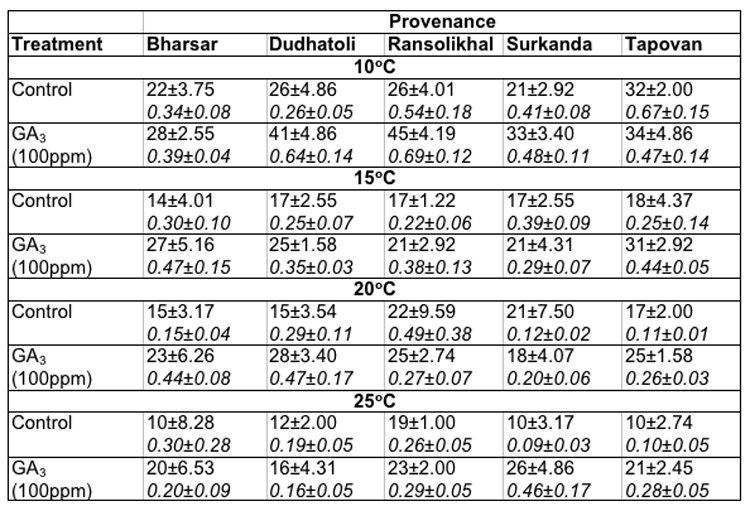
Table 2: Effect of treatment and temperature on seed germination and germination value of different provenances of Abies pindrow.
In Cupressus torulosa maximum seed germination at 100C (42.0 ± 2.55%), 15°C (35.0 ± 6.72%), 20°C (39.0 ± 3.32%) and 25°C (31.0 ± 1.0%) was noticed in Pauri provenance. However, maximum GVs were also recorded for Pauri provenance in these temperatures. Contrary to this the minimum germination percentage (14.0 ± 1.0%) and germination value were recorded at 20°C in Mandal provenance (Table-3).
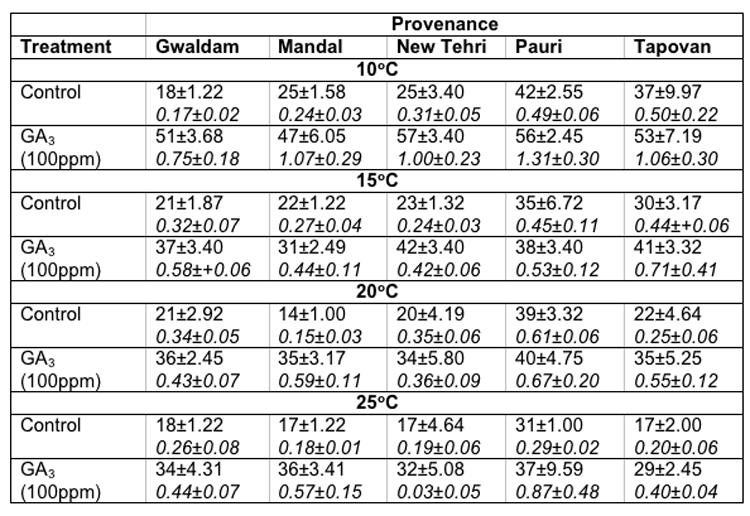
Table 3: Effect of treatment and temperature on seed germination and germination value of different provenances of Cupressus torulosa.
In Picea smithiana the maximum seed germination percentage oscillated significantly. For example, at 100C maximum seed germination (37.0 ± 2.25%) was recorded in Helang provenance. However, at 15°C (30.0 ± 1.58%), 20°C (30.0 ± 2.74%) and 25°C (26.0 ± 4.01%) the maximum seed germination was reflected by Pandukeshwar, Tpovan and Banjbagad provenances respectively. The minimum germination percentage of seeds (15.0 ± 5.71%) was found in Tapovan provenance at 25°C. On the other hand the maximum GV (1.26 ± 0.30 at 100C) was recorded for Pandukeshwar provenance, whereas, minimum (0.22 ± 0.10 at 25°C) for Tapovan provenance (Table-4).
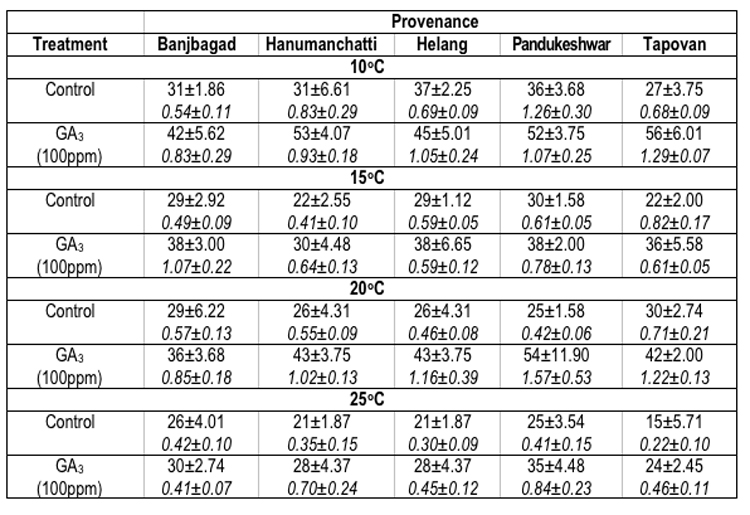
Table 4 : Effect of treatment and temperature on seed germination and germination value of different provenances of Picea smithiana.
Soaking of seeds in GA3: In Abies pindrow highest seed germination (45.0 ± 0.19%) and germination value (0.69 ± 0.12) were recorded for Ransolikhal provenance at 100C and minimum seed germination (28.0± 2.55%) and germination value (0.39 ± 0.04) for Bharsar provenance. At 15°C (31.0 ± 2.92%), 20°C (28.0 ± 3.40%) and 25°C (26.0 ± 4.86%) maximum germination percentages were recorded in Tapovan, Dudhatoli and Surkanda provenances. The GVs were almost highest in these provenances at these temperatures. The least germination value (0.16 ± 0.05) was recorded in Dudhatoli provenance at 25°C (Table-2).
In Cupressus torulosa maximum germination of seeds at 100C (57.0 ± 3.40%), and 15°C (42.0 ± 3.40%) was recorded in New Tehri provenance and at 20°C (40.0 ± 4.75%) and 25°c (37.0 ± 9.59%) in Pauri provenance. However, the minimum seed germination at 100C (47.0 ± 6.05%) and 15°C (31.0 ± 2.49%) was recorded in Mandal provenance, at 20°C (34.0 ± 5.80%) in New Tehri provenance and at 25°C (29.0 ± 2.45%) in Tapovan provenance respectively. The maximum GVs at 100C (1.31± 0.30), 20°C (0.67 ± 0.20) and 25°C (0.87 ± 0.48) were observed for Pauri provenance, however, at 15°C (0.71 ± 0.14) for Tapovan provenance. The overall minimum GV (0.03 ± 0.05) was found for New Tehri provenance at 25°C (Table-3).
In Picea smithiana, at 100C temperature, maximum seed germination (56.0 ± 6.01%) and GV (1.29 ± 0.07) were recorded for Tapovan provenance. At 150C Banjbagad (38.0 ± 3.00%), Helang (38.0 ± 6.65%) and Pandukeshwar (38.0 ± 2.0%) provenances have shown equal germination of the seeds, however maximum GV (1.07 ± 0.22) was observed for Banjbagad provenance. At 200C and 250C temperatures, maximum seed germination (54.0 ± 11.90% and 35.0 ± 4.48%) and GVs (1.57 ± 0.53 and 0.84 ± 0.23) were reflected by Pandukeshwar provenance. The overall minimum seed germination (24.0 ± 2.45% in Tapovan provenance) and GV (0.41± 0.07 for Banjbagad provenance) were found at 250C temperature in the seeds which were soaked in GA3 prior to germination at various temperatures (Table-4).
Thus, basing on the present study the Ransolikhal provenance of Abies pindrow, Pauri provenance of Cupressus torulosa and Pandukeshwar provenance of Picea smithiana were the most successful amongst all the provenances under the observed treatments. In all the selected temperature treatments (i.e., 100C, 150C, 200C & 250C), 100C was the best temperature for the germination of seeds in the selected species, as the highest germination was observed at this constant temperature, whereas, the least germination of seeds was recorded at 250C. On the other hand the seeds soaked with distilled water (as control) have exhibited lesser germination in all the provenances of A. pindrow, C. torulosa and P. smithiana. An increase in the seed germination of spruce, fir and Himalayan cypress by gibberellic acid treatment was observed probably due to enhancement of hydrolase (especially amylase) synthesis, as was also stated by Paleg (1960(a) & (b)), Amen (1968) and Galston & Davies(1969) or due to initiation of the embryo growth, as a result of which more gibberellic acid is synthesized by the growing embryo, which induced hydrolase synthesis ( Chen & Varner, 1973).
The data shown in Table-5 have revealed the significant effect of temperature and treatment on seed germination percent; however a non-significant effect of treatment x temperature interaction was also noticed. It was also observed that seed sources, which had higher values for cone and seed parameters, also showed better performance in germination. These findings have been supported by the concept of Baldwin (1942) and Dunlap & Barnett (1983), according to which, seed size and weight have pronounced effects on seed germination. Generally, large seeds have fast and uniform germination, due to more endosperm nutrient pool (Kandya, 1978). Therefore, seed source variation in germination percent and related traits may be ascribed to the significant differences, observed in seed dimensions and weight. Germination values varied considerably among seed sources and exhibited a random pattern, which is an index of combining speed and completeness of germination and itself is a function of seed size and weight ( Czabator, 1962) and ( Dunlop & Barnett, 1983). On the other hand, variation observed in time taken to complete germination could be attributed to the differences in germination rate and germination value of the selected provenances of all the three species.
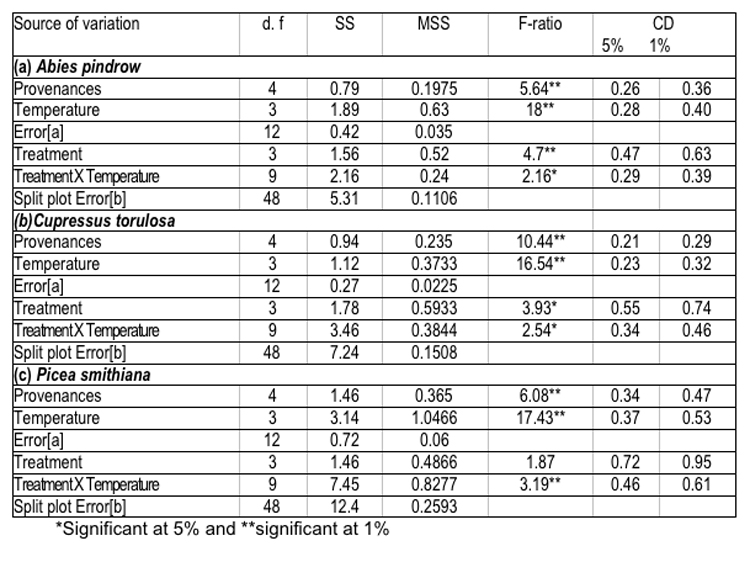
Table 5 : Analysis of variance for germination percent of various provenances of (a) Abies pindrow, (b) Cupressus torulosa and (c) Picea smithiana.
References 
[[source number="1"]]Amen, R. D. 1968. A model of seed dormancy. Botanical Review , 34 : 1-31
[[source number="2"]]Baldwin, H. I. 1942. Forest tree seeds of the north temperate region with special reference to North America. Chronica Botanica Co. Waltham, Mass. 240p
[[source number="3"]]Baskin, J. M. & C. C. Baskin. 1973. Plant population differences in dormancy and germination characteristics of seeds: heredity or environment Am. Midl. Nat., 90: 493-498
[[source number="4"]]Chandra, J. P. & P. S. Chauhan. 1976. Note on germination of spruce seeds with gibberellic acid. Indian Forester , 102(10): 721-725
[[source number="5"]]Chen, S. S. C. & J. E. Varner. 1973. Hormones and seed dormancy. Seed Science and Technology , 1(12): 325-338
[[source number="6"]]Czabator, F.J. 1962. Germination value: an index combining speed and completeness of pine seed germination. Forest Sciences 8(4): 386-396
[[source number="7"]]Dunlap, J. R. & J. P. Barnett. 1983. Influence of seed size on germination and early development of loblolly pine (Pinus taeda L.) germinants. Canadian Journal of Forest Research , 13: 40-44
[[source number="8"]]Galston, A.N. & P.J. Davies. 1969. Harmonal regulation in higher plant. Science N.Y. , 163: 1288-1297
[[source number="9"]]Kandya, A. K. 1978. Relationship among seed weight and various growth factors in Pinus oocarpa seedlings. Indian Forester , 104(8): 561-567
[[source number="10"]]Krishnamurthy, H. N. 1973. Gibberellins and plant growth. Wiley Eastern Limited, New Delhi, 356: 19-114
[[source number="11"]]Paleg, L.G. 1960a. Physiological effects of gibberellic acid I. on carbohydrate metabolism and amylase activity of barely endosperm, Plant Physiology , 35: 293-299
[[source number="12"]]Paleg, L.G. 1960b. Physiological effects of gibberellic acid II. On starch hydrolysing enzymes of barely endosperm. Plant Physiology , 35 : 902-906
[[source number="13"]]Rowe, J. S. 1964. Environmental preconditioning with special reference to forestry. Ecology, 45: 399-403
[[source number="14"]]Shafiq, Y. 1980. Effect of gibberellic acid (GA3) and pre-chilling on germination per cent of Nothofagus obliqua (Mirb.)Oerst. And N. procera Oerst. Seeds. Indian Forester , 106 (1): 27-33
[[source number="15"]]Singh, R.V. 1973. Regeneration of silver fir (Abies pindrow) forests. Proceedings on First Forest Conifers. , F.R.I., Dehradun
[[source number="16"]]Vogt, A. R. 1970. Effect of gibberellic acid on germination and initial seedlings growth of Northern oak. Forest Sciences , 16(4): 453-459
[[List of special symbols]]
±; plus/minus symbol. °C; Degree Celsius symbol. √; Square root symbol.