Abstract 
Vegetative and reproductive phenology of 57 overstorey
and 33 understorey species was studied in a tropical moist
deciduous forest of Similipal Biosphere Reserve (SBR) located
in Orissa state in India. A prominent peak in leaf drop, leaf flush
and flowering of overstorey species occurred in March, April and
April to May, respectively. However the peak period of such
phenological events in understorey species is slightly different than
over storey species. The peak fruiting period of both overstorey and
understorey species are same i.e. from May to June. The fruiting
phenology follows closely the flowering phenology. Fruit fall
culminates before or just at the beginning of the monsoon season and,
thus, ensures availability of sufficient moisture to seeds for
germination and seedling establishment. Leaf drop, leaf flush and
flowering both in overstorey and under storey species have been
triggered by changes in day length and temperature, which indirectly
signifies that soil moisture availability may have shaped the
phenological patterns of both overstorey and understorey species. The
phenological information obtained both for overstorey and understorey
species in the present study is mostly influenced by the seasons and
would be useful for planning proper management strategies in
Similipal biosphere reserve to sustain regeneration development .
Key words: Degree of
disturbance, Fruiting, Flowering, Leaf flushing, Phenological
activity, Seasonality.
Introduction 
Phenology is the study of the timing of recurring biological events, among phases of the plant species, which provide a background for collecting and synthesizing detailed quantitative information on rhythms of plant communities. Tropical plants with their high level of species diversity display phenological events such as leaf drop, leaf flushing, flowering and fruiting, etc. in relation to time and space (Justiniano and Fredericksen, 2000; Singh and Singh, 1992). Study of such events is useful in evolving proper management strategy as well as better understanding of natural forest regeneration potential and community level interactions (Fox, 1976). Studies from different parts of the world have shown that climatic factors are mainly responsible for vegetative and reproductive phenology at both community and species level. But phenology of the tropical forest tree species is not well understood although water stress is most frequently cited as a primary factor responsible for the timing of phenological events (Singh and Singh, 1992). However , various phenological events are triggered by rainfall, water availability, temperature, photoperiods, duration of dry spell and change in day length (Bhat and Murali, 2001; Hamann, 2004; Sivaraj and Krishnamurthy, 2002).
The tropical forests have a distinctive array of species different from temperate and rain forests. It supports different varieties of overstorey and understorey plant species, which are major food resources for a variety of biota (Bhat and Murali, 2001). In India extensive reports are available on phenological studies of tropical tree species in forest ecosystems of Central Himalaya (Ralhan et al., 1985a, b; Sundriyal, 1990), northeastern India (Shukla and Ramakrishnan, 1982; Kikim and Yadav, 2001), Western Ghats (Bhat and Murali, 2001) and Eastern Ghats (Sivaraj and Krishnamurthy, 2002). However the phenological pattern of tree species of Similipal Biosphere Reserve(SBR), which is included under Eastern Ghats has not yet been worked out. Therefore the present study aims at analyzing the phenological pattern of tree species to understand their response to climatic factors and the periodicity of seasons.
Description of study sites
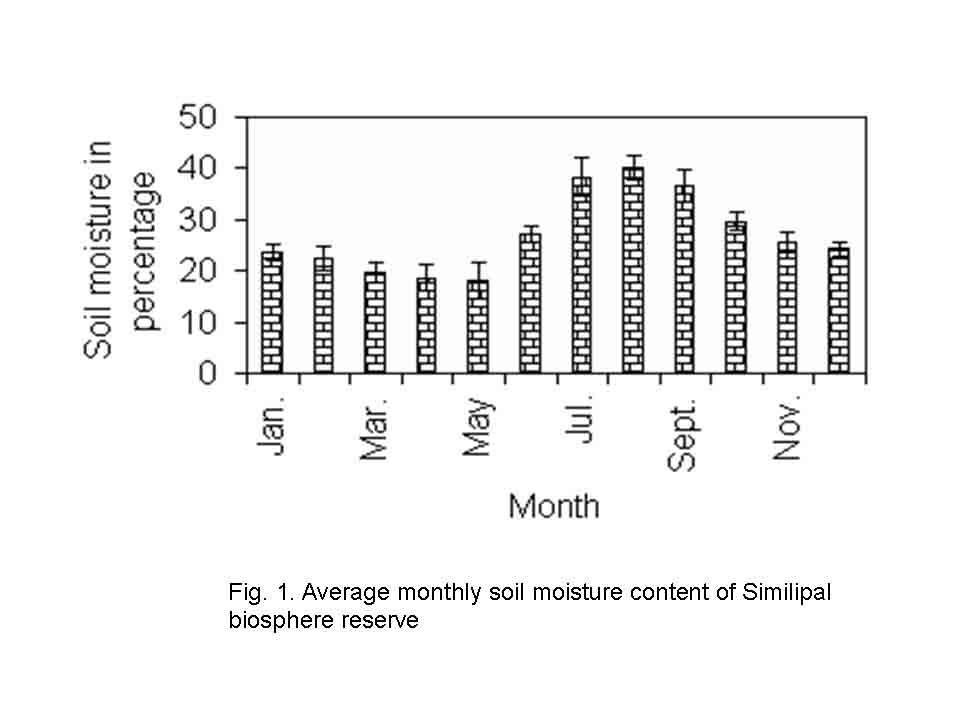
The study was conducted in northern tropical moist deciduous forests of Similipal in Mayurbhanj district of Orissa (210 28/ to 220 08/ N latitude and 860 04/ to 860 37/ E longitude). It is a tract of hilly terrain with gentle slopes and broad valleys at altitudes ranging from 80 to 869m. The soil of all the forest sites is reddish in colour and loam to sandy loam in texture. The soil is slightly acidic in nature with pH ranging from 5.23 to 6.52 and average monthly soil moisture content varies from 18.13 to 40.25 % (Fig.1).
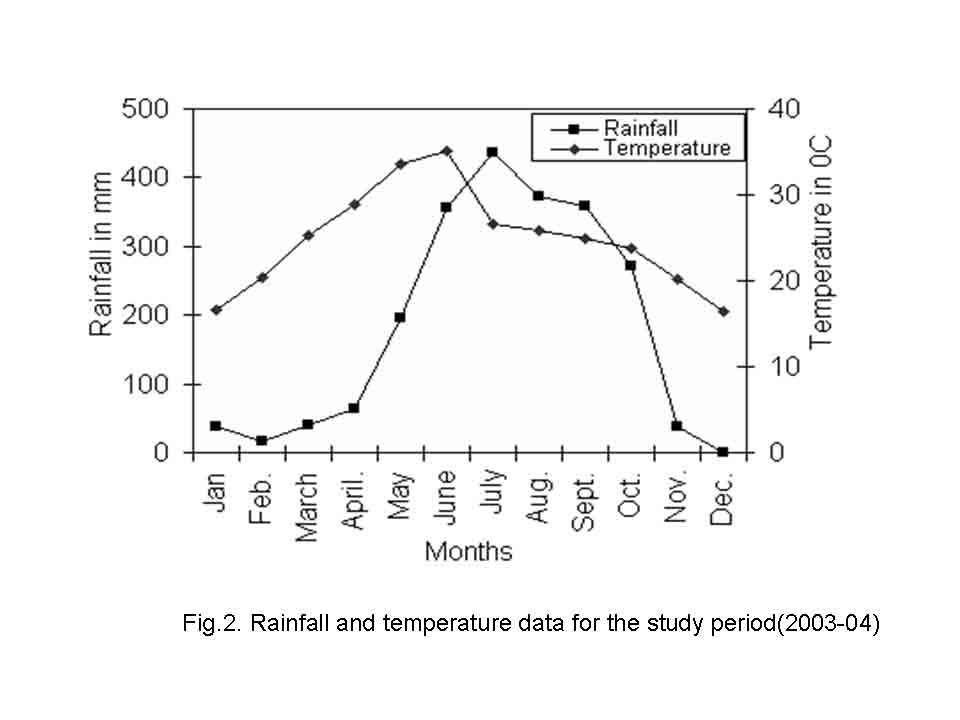
The climate of the area is monsoonal and divisible into three seasons; summer (March-June), rainy (July- October) and winter (November-February). The climatic description is based on temperature and rainfall. The average annual rainfall varies from 28.11 to 395.96 mm, and is largely restricted to the period from July to October. Pre-monsoon showers are received during May and June. Post monsoon showers are received during November and December. The mean maximum temperature varies from 16.39 C (December) to 35.03 C (June) and mean minimum temperature from 5.7 C (January) to 21.57 C (June) (Fig.2). The natural vegetation is moist deciduous type (Champion and Seth, 1968) and is dominated by Shorea robusta, Anogeissus latifolia, Buchnania lanzan, Dillenia pentagyna, Syzygium cumini and Terminalia alata. Saxena and Brahmam (1989) have given details of the floristic of SBR. Table 1 summarizes the salient features of the study sites.
Table 1: Characteristic features of
study sites
| Site | Elevation | Aspect | Density (Plants/ha) | Basal area (m2/ha) | Normal trees | Damaged trees | Total (Normal +Damaged) | D.I. (%) | L.I. |
| Podadiha | 80 | South-East | 680 | 52.36 | 109 | 84 | 193 | 43.52 | HB |
| Bangirposi | 226 | East | 675 | 59.54 | 107 | 81 | 188 | 43.68 | HB |
| Handipuhan | 280 | West | 715 | 48.71 | 111 | 96 | 207 | 46.38 | HB |
| Ghodabindha | 557 | West | 650 | 49.13 | 105 | 82 | 187 | 43.85 | HB |
| Chahala | 774 | North | 875 | 88.59 | 168 | 29 | 197 | 14.72 | NB |
| Nigirdha | 830 | North | 985 | 85.07 | 190 | 38 | 228 | 16.67 | NB |
| UBK* | 824 | South | 970 | 84.86 | 186 | 36 | 222 | 16.22 | NB |
| Jenabil | 869 | South | 895 | 104.92 | 175 | 31 | 206 | 15.05 | NB |
L.I. - Level of Interference
D.I. (Disturbance Index) = Percentage of damaged individuals of the total number of woody individuals per 2000 m2 area.
*UBK- Upper Barakamda
HB- High biotic interference; NB- No biotic interference
Materials and Methods 
Eight one-hectare permanent study plots were selected inside the Similipal Biosphere Reserve. Four of the eight one-hectare plots were situated in buffer zone while the other four were situated in the biotically undisturbed zone (core zone). All the overstorey and understorey woody plants having cbh (Circumference at breast height, 137cm) of > >31cm were enumerated in each study plot. The plants were identified following Saxena and Brahmam (1994-96) and Haines (1925). Phenological observations were made on 90 species from eight sites along biotically disturbed and undisturbed gradient, determined on the basis of protection afforded to each forest site, which is reflected through the tree density, basal area and disturbance index of each study site. Individuals of each of the 90 species were marked and tagged for each species. Whenever the required number of individuals was not available inside the permanent plot, additional individuals in adjacent area were also marked. Besides, observations on large populations by taking additional similar stands were also made to alleviate replication deficiency. If a given phenophase was observed in 5-10 percent individuals of a species, it was considered to have initiated. For each tagged tree, records were made on leaf drop, leaf flushing, flowering and fruiting. The duration of activity and phenological behaviour of tree species were determined following the method given by Opler et al. (1980).
The phytosociological characters such as density and basal area of individual species were quantified using standard quantitative methods (Muller-Dombois and Ellenberg, 1974). For measuring disturbance index, the physical condition of each individual tree present inside the 10 x 10 m2 quadrat was noted under normal and damaged categories. The trees involved under normal categories were the healthy individuals. The damaged category included the individuals that were partly broken at the top, partly dry or were green fallen. The individuals that were standing dead, dead cut stump and completely dry were also taken into consideration. Because such plants create problems in identification only numbering was done. All tree species were divided into two categories: (i) Overstorey species consists of canopy and subcanopy trees with a height of > > 10m and (ii) understorey species with < < 10m. Evergreen species continually produce at least small amount of new leaves throughout the year and do not show heavy leaf fall at a concentrated period whereas deciduous species has a marked leaf fall and leaf flushing at a concentrated period of the year.
Duration and pattern of activity
Both brief and extended activity was observed for the periodicity of leaf flushing, flowering and fruiting activity by individuals of a species population. Brief activity extends for 2 weeks or less while extended activity refers to periods more than 2 weeks. A more or less continuous flowering and fruiting activity throughout the year is referred to as continuous activity. The term seasonal and extended activity refers to flowering/fruiting occurs during a given period and extending into more than one period respectively. Marginal activity refers to species that have their activity occurring during transition period of seasonal changes. When some individuals of a tree species are in flowering/fruiting simultaneously is referred to as synchronous activity (S). The species showing flower/fruit development during a distinct period is known as asynchronous (A).
Fruit maturation activity
There are 2 categories of fruiting activity i.e. rapid and lengthy. Rapid (r) can be characterized as fruit maturation periods of 4 months or less following fertilization and those more than 4 months is termed as lengthy (L).
Results and Discussion 
Out of 90 species, 52 were deciduous (32 overstoreys and 20 understoreys), 21 were semi-evergreen (17 overstoreys and 4 understoreys) and 17 were evergreen species (8 overstoreys and 9 understoreys). The forest does not maintain its green appearance throughout the year because majority of the species are deciduous in this forest. However in the wet months of July to October deciduousness of the forest is not so conspicuous due to reduced leaf fall in comparison to the drier and cool months. The general phenological stages of all species are presented in Table 2 (over storey species) and Table- 3 (under storey species). Their seasonal phenological behaviours have been discussed under leaf drop, leaf flushing, flowering and fruiting activities.
Table 2: Phenology of overstorey woody tree taxa of SBR
| Name of the plant species | VT | Leaf drop | Leaf flushing | Flowering | Fruiting |
| Anogeissus latifolia | D | 3 (r) A | 4 (e) | 6-9 (eA) | 12-1, 3 (eRM) |
| Adina cordifolia | D |
2-3 ( r ) A
|
4 (e) | 6-7 (eA) | 2-5 (eR) |
| Aegle marmelos | D | 3-4 (r ) A | 5 (e) | 3-4 (eA) | 5-6 (eR) |
| Albizia procera | SE | 5-6 (r) S | 6(e) | 8-9(eA) | 12,1,2,3,4,5(eL) |
| Albizia marginata | E | 1-2 (r) A | 3(e) | 5-6(eA) | 10-4 (eL) |
| Albizia lebbeck | D | 1 (r) A | 4(e) | 2-4(eS) | 3-6(eR) |
| Alangium salvifoila | D |
1-2 (r) A |
3(e) | 2-5(eS) | 3-5(eR) |
| Anthocephalus cadamba | E | 1-12/3-4*(l) S | - | 5-7(eA) | 8-10(eR) |
| Buchanania lanzan | D | 12,1 (r) S | 1(e) | 2-3(eS) | 3-4(eR) |
| Bridelia retusa | E | 1-12/3-4*(l) S | - | 8-10(eA) | 11,12,1(eR) |
| Bombax ceiba | D | 2-3 (r ) A | 4(e) | 3-4(eS) | 3-6(eR) |
| Baringtonia acutangula | SE | 2-3 (r) A | 4(e) | 5(eA) | 9(eR) |
| Croton roxburghii | D | 1-2 (r) A | 3(e) | 1-2(eA) | 3-4(eR) |
| Careya arborea | D | 2-3 (r) S | 3(e) | 3-5 (eA) | 7 (eR) |
| Cassia fistula | D | 3-4 (r) S | 4 (e) | 5-8 (eA) | 1-12/4* (eL) |
| Dalbergia sisoo | D | 12,1,2,3 (l) A |
4 (e) |
3-4 (eA) | 6-7 (eR) |
| Diospyros melanoxylon | D | 1-2 (r) A | 3 (e) | 5 (eS) | 5-7 (eR) |
| Dalbergia latifolia | D | 4-6 (l) S |
6 (e) |
9 (eA) | 1-2 (eR) |
| Dillenia pentagyna | D | 2.5-5(l) S | 5(e) | 3-4 (eA) | 5-6 (eR) |
| Diospyros embryopteris | E | 1-12/3-4* (l) S | 4 (e) | 5 (eS) | 3-6 (eR) |
| Diospyros malabarica | E | 1-12/2-3* (l) S | 4 (e) | 3-4 (eA) | 5 (eR) |
| Diospyros sylvatica | SE | 3-4 (r) A | 4 (e) | 5 (eA) | 1-3 (eR) |
| Diospyros Montana | D | 1-2 (r) S | 2 (e) | 4-6 (eA) | 12 (eR) |
| Ficus benghalensis | SE | 3 (r) A | 5-6 (e) | 5-8 (eS) | 4-6,12-2 (eRM) |
| Ficus hispida | SE | 3 (r) A | 4-5 (e) | 1-12 (eS) | 10-12 (eR) |
| Ficus microcarpa | SE | 3 (r) S | 3-4 (e) | - | 10-4 (eL) |
| Ficus religiosa | D | 12-2 (l) A | 3-4 (e) | - | 6-10 (eL) |
| Gmelina arborea | D | 2-4 (l) S | 4 (e) | 2-4 (eA) | 5-6 (eR) |
| Garuga pinnata | SE | 1-2 ( r) S | 2-3 (e) | 2-3 (eS) | 3-5 (eR) |
| Kydia calycina | D | 3 (r) S | 3.5 (b) | 9-11 (eA) | 12-5 (eL) |
| Lannea corromandelica | D | 3-4 ( r) S | 4 (e) | 3-4 (eS) |
-
|
| Lagerostroemia parviflora | D | 2-3 (r) S | 3 (e) | 4-5 (eA) |
12-1 (eR)
|
| Madhuca indica | D | 3-4 (r) S | 4 (e) | 3-4 (eA) | 6-7 (eR) |
| Mangifera indica | SE | 4-5 (r) A | 6 (e) | 1-3 (eA) | 5-6 (eR) |
| Mitragyna parviflora | SE | 4-6 (l) S | 4-6 (e) | 5-6 (eA) | 3-4,11 (eRM) |
| Melia dubia | D | 3-4 (r) A | 5 (e) | 6 (eS) | 6 (eR) |
| Miliusa velutina | D | 4(r) A | 5-6 (e) | 5-6 (eS) | 6-7 (eR) |
| Michelia champaca | SE | 3-5 (l) S | 3,10 (M) | 4-5 (eA) | 7 (eR) |
| Pterocarpus marsupium | SE | 4-5 (r) S | 5-6 (e) | 10 (eA) | 12-1 (eR) |
| Protium serratum | D | 3-4 (r) S | 4(e) | 4 (eA) | 5-8 (eR) |
| Phoebe wightii | SE | 3 (r) A | 4 (e) | 4.5 (bS) | 5-6 (eR) |
| Phoebe lanceolata | SE | 2-3 (r) A | 4(e) | 4-5 (eS) | 5-6 (eR) |
| Shorea robusta | D | 3(r) A | 4 (e) | 3-4 (eA) | 6-7 (eR) |
| Syzygium cumini | SE | 2-3 (r) A | 4 (e) | 3-4 (eA) | 5-7 (eR) |
| Syzygium cerasoides | SE | 3-4 (r) S | 4 (e) | 5-6 (eA) | 8 (eR) |
| Sterospermum suaveolens | D | 3 (r) A | 4 (e) | 4-5 (eA) | 9-2 (eL) |
| Schleichera oleosa | SE | 3(r) A | 4 (e) | 3 (eA) | 6 (eR) |
| Suregada angustifolia | E | 1-12/1-2* (l) S | 2 (e) | 3-4 (eA) | - |
| Samanea saman | E | 1-12 (l) S | - | 3-4 (eA) | 5-7 (eR) |
| Schrebra swietenoides | SE | 2-3 (r) A | 4-5 (e) | 4-5 (eS) | 5-6 (eR) |
| Terminalia alata | D | 3-5(l) A | 6-7 (e) | 5-6 (eA) | 7-10 (eR) |
| Terminalia chebula | D | 2-3 (r) A | 4 (e) | 4-5 (eA) | 11-2 (eR) |
| Terminalia bellirica | D | 2-3 (r) A | 4,10 (eM) | 3-5 (eA) | 1-2 (eR) |
| Trewia nudiflora | D | 12,1-2 (l) A | 3 (e) | 1-3 (eA) | 10-12 (eR) |
| Terminalia arjuna | D | 3(r) A | 4 (e) | 4-7 (eS) | 5-8 (eR) |
| Vitex leucoxylon | E | - | - | 5-6, 10 (eAM) | 2 (eR) |
| Xylia xylocarpa | D | 4-5 ( r) S | 5 (e) | 4-5 (eS) | 2-4 (eR) |
V

 T
vegetation type; 1-12: January to December; r = rapid leaf drop <
< 2 months; l = lengthy leaf drop > > 2 months; * =
concentrated period of leaf drop; e = extended flowering/fruiting
extending into more than one period; S (synchronous)=
flowering/fruiting taking place simultaneously; A (a synchronous)=
Flower/Fruit development during distinct period; D= deciduous; E=
evergreen; SE= semi-evergreen; b= brief periods < < 2 weeks
per episode; e= extended periods > > 2 weeks per episode;
M= multiple events per year; R= rapid fruit maturation < > 4 months.
T
vegetation type; 1-12: January to December; r = rapid leaf drop <
< 2 months; l = lengthy leaf drop > > 2 months; * =
concentrated period of leaf drop; e = extended flowering/fruiting
extending into more than one period; S (synchronous)=
flowering/fruiting taking place simultaneously; A (a synchronous)=
Flower/Fruit development during distinct period; D= deciduous; E=
evergreen; SE= semi-evergreen; b= brief periods < < 2 weeks
per episode; e= extended periods > > 2 weeks per episode;
M= multiple events per year; R= rapid fruit maturation < > 4 months.Table-3: Phenology of understorey woody tree species of
SBR
| Name of the plant species | VT | Leaf drop | Leaf flushing | Flowering | Fruiting |
| Artocarpus lacucha | SE | 3 (r) A | 4 (e) | 12,4 (eA) | 5,10-11 (eRM) |
| Alangium salvifolium | D | 10-11 (r) A | 1-2 (e) | 1-3,12 (eSM) | 1-5 (eL) |
| Bauhinia malabarica | E |
1-2
(r) A
|
3 (e) | 3-6 (eS) | 5-11 (eL) |
| Bauhinia purpurea | SE | 4 (r) A | 6 (e) | 7-8 (eA) | 10-11 (eR) |
| Bauhinia variegata | E | 3 (r) A | 4 (e) | 2-3 (eA) | 4-5 (eR) |
| Crateva religiosa | D | 1-3 (r) S | 3 (e) | 3-4 (eA) | 6 (eR) |
| Casearia graveolens | D | 5-6 (r) S | 6 (e) | 5-6 (eS) | 5-7 (eR) |
| Cartunaregum spinosa | D | 3-4 (r) A | 5 (e) | 4-5 (eA) | 9-12 (eR) |
| Chionanthus intermedius | D | 3-4 (r) S | 5 (e) | 1-4 (eS) | 3-5 (eR) |
| Casearia elliptica | D | 2-4 (r) S | 4 (e) | 2-5 (eS) | 4-5 (eR) |
| Cleistanthus collinus | D | 3-4 (r) A | 4 (e) | 4-5,9 (eAM) | 3-4 (eR) |
| Euonymus glaber | E | - | - | 5 (eAS) | 3-6 (eR) |
| Ficus glomerata | D | 10-11 (r) A | 12-1 (e) | 4 (eS) | 3-6 (eR) |
| Glochidion velutinum | D | 3-4 (r) S | 4-5 (e) | 4-5 (eA) | 6-8 (eR) |
| Glochidion lanceolarium | E | - | 3 (e) | 3-5 (eA) | 9-1 (eL) |
| Gardenia latifolia | D | 3-4 (r) A | 5 (e) | 4 (eA) | 12-6 (eL) |
| Gardenia gummifera | D | 4 (r) A | 5 (e) | 3-5 (eA) | 6-8 (eR) |
| Homalium nepalens | D | 3-4 (r) A | 5 (e) | 5-6 (eA) | - |
| Hymenodictyon excelsum | D | 11-5 (l) S | 5 (e) | 8 (eA) | 1 (eR) |
|
Holarrhena
antidysentrica |
D | 2-4 (l) S | 4 (e) | 5-7 (eA) | 12-2 (eR) |
| Hyptianthera sticta | E | 1-12 (l) S | - | 4-8 (eA) | 11-2 (eR) |
| Ixora parviflora | E | 1-12 (l) S | - | 3-5 (eS) | 5-6 (eR) |
| Ligustrum gamblei | SE | - | - | 6-8 (eA) | 12-1 (eR) |
| Nyctanthes arbor-trstis | D | 4-5 (r) S | 5 (e) | 9-11 (eA) | 12-1 (eR) |
| Ochna obtusata | D | 2-3 (r) A | 4 (e) | 3-5 (eA) | 6-8 (eR) |
| Phyllanthus emblica | D | 4 (r) A | 5 (e) | 9 (eS) | 9 (eR) |
| Prunus ceylanica | SE | 9-10 (r) A | 11 (e) | 8 (eA) | 11-2 (eR) |
| Securinega virosa | D | 3-4 (r) S | 4 (e) | 5-9 (eS) | 5-9 (eL) |
| Symplocos cochinchinensis | E | 1-12/5-6*(r) S | 2-3 (e) | 1-3 (eA) | 4-6 (eR) |
| Wendlandia tinctoria | E | 1-12/1-2* (r) S | 3-4 (e) | 9-10 (eA) | 3-6 (eR) |
| Wendlandia exerta | E | 1-12/2-3* (r) S | 4-5 (e) | 1-3 (eS) | 3-4 (eR) |
| Ziziphus rugosa | D | 1-3 (l) A | 5 (e) | 3-4 (eS) | 4-5 (eR) |
| Ziziphus mauritiana | D | 1-2 (r) A | 4 (e) | 2-3 (eA) | 5-7 (eR) |
V

 T
vegetation type; 1-12: January to December; r = rapid leaf drop <
< 2 months; l = lengthy leaf drop > > 2 months; * =
Concentrated period of leaf drop; e = extended flowering/fruiting
extending into more than one period; S (synchronous)=
flowering/fruiting taking place simultaneously; A (a synchronous)=
Flower/Fruit development during distinct period; D= deciduous; E=
evergreen; SE= semi-evergreen; b= brief periods < < 2 weeks
per episode; e= extended periods > > 2 weeks per episode;
M= multiple events per year; R= rapid fruit maturation < > 4 months.
T
vegetation type; 1-12: January to December; r = rapid leaf drop <
< 2 months; l = lengthy leaf drop > > 2 months; * =
Concentrated period of leaf drop; e = extended flowering/fruiting
extending into more than one period; S (synchronous)=
flowering/fruiting taking place simultaneously; A (a synchronous)=
Flower/Fruit development during distinct period; D= deciduous; E=
evergreen; SE= semi-evergreen; b= brief periods < < 2 weeks
per episode; e= extended periods > > 2 weeks per episode;
M= multiple events per year; R= rapid fruit maturation < > 4 months.Leaf drop
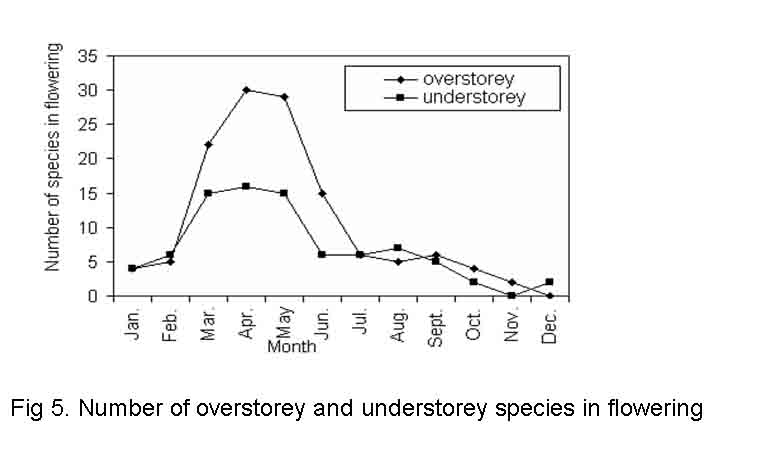
Leaf drop may be total or partial depending upon the species. In some truly deciduous taxa, all or most of the old leaves got abscised before the arrival of new ones and the tree was bare for a period of weeks or to few months. Examples of this category are Anogeissus latifolia, Adina cordifolia, Aegle marmelos, Albizia marginata, Bombax ceiba, Croton roxburghii, Diospyros melanoxylon, etc. In other species such as Ficus benghalensis, Albizia procera, Barringtonia acutangula, Diospyros sylvatica, Garuga pinnata, Ficus microcarpa, Mangifera indica and Mitragyna parviflora, leaf fall and leaf flushing processes slightly over lapped in the same tree. In evergreens old leaves were abscised over a period of time throughout the year, thus, retaining a steady population of functional leaves all the time. Majority of the species start leaf shedding in dry months i.e. from January and extending up to May and being low in other months. The peak of leaf drop was recorded in February to March in overstorey species and March to April in understorey species (Fig.3). In overstorey species the leaf drop was significantly (F = 23.906, P< < 0.001) different among the seasons but insignificant between the sites. However, in understorey species the variation of leaf drop was significant both among the seasons and sites (F = 29.835, P < < 0.001; F = 12.688, P< < 0.001 for season and site, respectively). Such phenological leaf drop in overstorey species in relation to aspects was insignificant but significant (F = 6.884, P< < 0.001) in understorey species (Tables 4 and 5). Again the study reveals that site, season and category have no interactive significant effect on leaf drop, while site, season and the degree of disturbance among the study sites for both the layers have interactive effects on leaf drop which is statistically significant (F = 4.104, P< < 0.05) (Table-6). Arjunan and Ponnammal (1993) stated that leaf drop is delayed due to rain and high temperature and advanced due to drought and low temperature. In the present investigation it was observed that the leaf drop of overstorey and understorey species had negative significant correlation with rain fall (r = -0.609 and -0.610 for overstorey and understorey species, respectively at p < > flowering after leaf flushing > > flowering before leaf flushing> > flowering later after leaf flushing. For understorey species the order was: flowering and leaf flushing simultaneously > > flowering later after leaf flushing > > flowering after leaf flushing = flowering before leaf flushing. The synchronization of flowering with leaf flushing seems to be related to moisture, temperature and photoperiod ( Bhooj and Ramkrishnan, 1981; Murali and Sukumar, 1994). Cool and dry winter period is responsible for maximum leaf drop whereas increase in temperature during warm and dry periods induces the leaf flushing and flowering in most of the species.
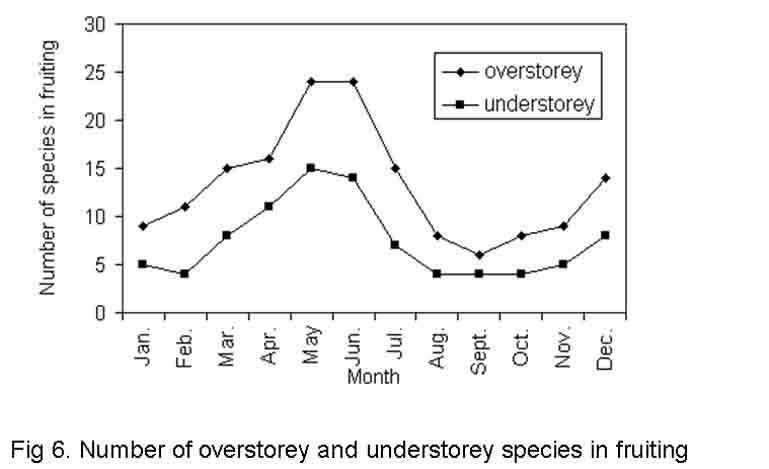
Fruiting activity
Fruiting activity was observed throughout the year with approximately 1.5 times more activity around May and June than in December (Fig.6). The peak period of fruit maturity in the present study was observed during winter and summer. The fruit development period for different species of both layersvaries from 4 to 28 weeks. A majority of the species in both the categories showed rapid fruiting activity. Next to rapid fruiting activity a larger proportion of species recorded lengthy fruiting behaviour but only very few species had multiple fruiting behaviour (Table-2 and 3). Almost all tree species had phenological patterns that synchronized flowering and fruiting in the dry months i.e. April, May and June. Most of the species flowered at the beginning of April and fruited near the end of May and beginning of June, needing only a short time for the development of fruits. Rest of the species flowered during April and May, fruited during December, with a moderate amount of time required for fruit development. Flowering and fruiting at hottest summer i.e. April and May have selective advantage. It is more efficient to transfer assimilates directly into growing organs rather than having to store them and mobilize and translocate them latter (Wright and Schaik, 1994). We observed strong positive correlations (r =0.962 and 0.963 at P < < 0.001 for overstorey and understorey tree species, respectively) between the temperature of hottest months and the number of species fruited in the same period. This perhaps establishes that increased temperature favours formation of fruits in most of the overstorey and understorey species. Analysis of variance of fruiting activity between site, category and season was found significant (F= 2.278, 19.97 and 138.21 at P < < 0.05, 0.001 and 0.001 for site, season and category, respectively). But such phenological parameter has no interactive effect between site, season and category. However, season, category and the degree of disturbance among the study sites have interactive significant effect (F = 5.214) at P< < 0.01 (Table 6).
Table 6: Analysis of variance of different phenological
conditions of overstorey and understorey woody tree species of
SBR.
| Phenological condition | F-value between parameters | |||||
|
|
Season | Site | Category | Disturbed and undisturbed | Season X Site X category | Season X Site X Disturbed and undisturbed |
| Leaf drop | 22.383*** | 1.133 (NS) | 19.509 *** | 0.00069 (NS) | 0.368 (NS) | 4.104* |
| Leaf flush | 14.68*** | 19.54*** | 3.29 (NS) | 0.00048 (NS) | 2.09 * | 7.875 ** |
| Flowering | 96.69*** | 1.654 (NS) | 81.021*** | 0.084 (NS) | 2.189* | 21.675*** |
| Fruiting | 19.97*** | 2.278* | 138.21*** | 0.590 (NS) | 1.354 (NS) | 5.214** |
***- Significant at P< < 0.001
**- Significant at P < < 0.01
*- Significant at P < < 0.05
In both the category of woody tree species ripening of fruits began in the later part of rainy season and continued upto end of cool and dry winter period. This is due to the difference in time taken for fruit maturation. Forty-seven out of 57 overstorey species showed extended and rapid fruiting activity, while only 7 and 3 species showed extended lengthy and extended rapid and multiple fruiting activity, respectively. Similarly out of 33 understorey species 27, 5 and one species showed extended rapid, extended lengthy and, extended and multiple fruiting activity, respectively. Similar observation has also been reported for tropical forests (Bullock and Solis Margallenus, 1990; Frankie et al., 1974) of Himalaya. It has also been reported that minimal pest pressure (Aide, 1988) and maximal activity of pollinating insects (Foster, 1996) may occur during dry season. Also fruit production at the end of dry season ensures that seedlings are not immediately exposed to water stress.
Acknowledgements 
The authors are thankful to Council of Scientific and Industrial Research (CSIR) and Department of Science and Technology (DST), New Delhi for providing financial Assistance to carry out the research work.
17
References 
Aide, T.M. 1988. Herbivory as selective agent on the timing of leaf production in a tropical understorey community. Nature, 336: 574-575.
Arjunan, M.C. & N.R. Pannammal. 1993. Studies on phenology and nursery technology of certain tree species. Journal Indian Botanical Society, 10: 147-150.
Bhat, D. M.& Murali, K. S. 2001. Phenology of understorey species of tropical moist forest of Western Ghats region of Uttara Kannada district in south India. Current Science, 81(7): 799-805.
Boojh, R. & P.S. Ramakrishnan. 1981. Phenology of trees in a subtropical evergreen montane forest in northeast India. Geo-Eco-Trop., 5: 189-209.
Borchert, R. 1994. Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology, 75: 1437-1449.
Bullock, S. H. & Solis Margallenus, J. A. 1990. Phenology of canopy trees of a tropical deciduous forest in Mexico. Biotropica, 22: 22-35.
Champion, H.G. & S.K. Seth. 1968. A revised Survey of the Forest Types of India. Govt. of India Press, Delhi.
Frankie, G.W.; H.G. Baker & P.A. Opler. 1974. Comparative phenological studies of trees in tropical wet and dry forest in the low lands of Costa Rica. Journal of Ecology, 62: 881-913.
Fox, J.E.D. 1976. Constraints on the natural regeneration of tropical moist forests. Forest Ecology and Management, 1: 37-65.
Foster, R.B. 1996. The seasonal rhythm of fruit fall in Barro Colorado Island. Pp. 7-12 in Leigh, E.G. Jr. (Ed.). The Ecology of a Tropical Forest. 2nd Edition. Smithsonian Inst. Press, Washington, DC.
Haines, H.H. 1921-1925. The Botany of Bihar and Orissa. Vol. I-III. London, Botanical Survey of India, Calcutta (Repn. Edn. 1961).
Hamann, A. 2004. Flowering and fruiting phenology of a Philippine submontane rainforest: climatic factors as proximate and ultimate causes. Journal of Ecology, 92: 24-31.
Justiniano, M.J. & T.S. Fredericksen. 2000. Phenology of timber tree species in a Bolivian dry forest: implications for forest management. Journal of Tropical Forest Science, 12(1): 174-180.
Kikim, A. & P.S. Yadava. 2001. Phenology of tree species in subtropical forests of Manipur in northeast India. Tropical Ecology, 42(2): 269-276.
Muller-Dombois, D. & H. Ellenberg. 1974. Aims and Methods of Vegetation Ecology. John Wiley and Sons, New York.
Murali, K.S. & R. Sukumar. 1994. Reproductive phenology of a tropical dry forest in Mudumalai, Southern India. Journal of Ecology, 82: 759-767.
Opler, P.A.; G.W. Frankie & H.G. Baker. 1980. Comparative phonological studies of shrubs and treelets in wet and dry forests in the lowlands of Costa Rica. Journal of Ecology, 68: 167-186.
Ralhan, P.K.; R.K. Khanna; S.P. Singh & J.S. Singh. 1985a. Phenological characteristics of the tree layer of Kumaun Himalayan forests. Vegetatio, 60: 91-101.
Ralhan, P.K.; R.K. Khanna; S.P. Singh & J.S. Singh. 1985b. Certain phonological characters of the shrub layer of Kumaun Himalayan forests. Vegetatio, 63: 113-120.
Saxena, H.O. & M. Brahmam. 1989. The Flora of Similipahar (Similipal) Orissa. Regional Research Laboratory, Bhubaneswar.
Saxena, H.O.& M. Brahmam. 1994-1996. The Flora Of Orissa. Vol. I-IV. Regional Research laboratory (CSIR), Bhubaneswar and Orissa Forest Development Corporation Ltd., Bhubaneswar.
Shukla, R.P.& P.S. Ramakrishnan 1982. phenology of trees in a subtropical humid forest in northeastern India. Vegetatio, 49: 103-109.
Singh, J.S. & V.K. Singh, V.K. 1992. Phenology of seasonally dry tropical forest. Current Science, 63(11): 684-689.
Sivaraj, N. & K.V. Krishnamurthy. 2002. Phenology and reproductive ecology of tree taxa of Shervaroy Hills, Eastern Ghats, south India. Proceedings of National Seminar on Conservation of Eastern Ghats, Tirupati, march, 24-26.
Sundriyal, R.C. 1990. Phenology of some temperate woody species of the Garhwal Himalaya. International Journal of Ecology and Environmental Sciences, 6: 107-117.
Wright, S.J. & C.PV. Schaik. 1994. Light and phenology of tropical trees. American Naturalist, 143: 192-199.
15