Abstract 
For this project, the sequestration and storage
capacity of carbon were quantified in canopy trees along with their
epiphytes in a fragment of a cloud forest where oak trees - Quercus
humboldtii - represent the largest part of the whole forest. It
was found that each of the components of the epiphyte-host tree
system shows different percentages of C concentration. In the case of
Oak trees, it was found that the branches with diameters lower than 5
cm. have the largest capacity in capturing C (40.13%), followed by
the wood contained in the trunk and in branches with diameters higher
than 5 cm (38.75%) and fresh leaves and dead leaves show (35.95%) and
(34.05%) of C retention respectively. In the case of epiphytes
growing on these trees, it was discovered that the lichens and
bryophytes yielded a 43% of C, being the
component with more capacity of retention of C in the epiphyte -
host tree system; the bromeliads had a capacity of 38.82% also
presenting a bigger capacity of capture of C than that of the
components of their host tree, excepting the upper branches.
As for the C stored in the biomass it was
found that in the four evaluated trees and their epiphytes, there was
32 066 kg in 0.0938 ha.
Key words: Neotropic,
Cloud Forest, Carbon sequestration, Canopy, Oaks, Epiphytes
Resumen 
Se cuantificó la capacidad de captura y almacenamiento de
carbono en árboles de dosel y sus epífitas en un
fragmento de bosque de niebla dominado por árboles de Quercus
humboldtii. Se encontró que cada componente del sistema
epífitas - hospederos presenta diferentes porcentajes de
concentración de C, en el caso de los árboles de roble
se obtuvo que las ramas con diámetros inferiores a 5 cm. son
las que mayor capacidad de captura de C presentan (40.13%), seguido
por la madera contenida en el tronco y en las ramas con diámetros
mayores a 5 cm (38.75%), las hojas frescas (35.95%) y la hojarasca
(34.05%). En el caso de las epífitas que crecen sobre estos
árboles se encontró que los líquenes y briófitos
con el 43 % son el componente que más capacidad de retención
de C tuvieron en el sistema epífitas - hospederos; las
bromelias tuvieron una capacidad de 38.82% presentando también
una mayor capacidad de captura de C que la de los componentes de su
hospedero, exceptuando las ramas superiores. En cuanto al C
almacenado en la biomasa se encontró que en los cuatro árboles
evaluados y sus epífitas, hubo 32 066 kg en 0.0938 ha
Palabras claves:
Neotrópico, Bosque de niebla, Captura de carbono, Dosel,
Robles, Epífitas
Introduction 
Tropical forests and temperate zones sequester and store more carbon than any other terrestrial ecosystem does. In addition, these ecosystems contribute to the 90% of the annual flow of C between the atmosphere and the ground (Brown et al. 1993, Dixon et al. 1994). This fact has generated a special concern about the great importance of these forests as C stock places (Husch 2001), since they store big amounts of greenhouse gases, especially CO2 (Macera et al. 2000).
In the case of tropical forests, these have received special attention due to their wide covering on a great part of the terrestrial surface (Terborgh 1985), the rapid transformation rate into commercial plantations and pastures (Vitousek et al. 1987), and their contributions to the C cycles as well as their potential impacts in the global weather (Brown & Lugo 1982). In the last decades, the amount of C found in land vegetation has generated a special concern, so different conservation and reforestation strategies have being posed in order to face this situation (Macera et al. 2000).
Eleven percent of these tropical forests are represented by montane and submontane forests. They are spread all over America, Africa, Southwest of Asia, and Pacific islands (Doumenge et al. 1995). In America, Montane and submontane forests are located in Central America, the Caribbean (Labastille & Pool 1978) as well as in the tropical Andes in the northern part of South America (UNESCO 1981). In this continent, the biggest extension of montane forests is located in Peru, followed by Colombia, Bolivia, Ecuador, and Venezuela.
In Colombian submontane and montane forests, where generally a co-dominance is presented among several species, it can be found homogeneous woods dominated by Oaks - Quercus humboldtii- (Lozano & Torres 1974). These forests are located in the three Colombian mountain ranges, covering areas from 1 100m up to 3 450m high (Cavelier et al. 2001). In the past, these forests covered big areas of land. However, nowadays, the presence of the oak in Colombia is limited to discontinuous fragments that put it in certain threat degree (UNESCO 2001). So far, studies about the capacity of capturing carbon in canopy trees (Q. humboldtii) and their epiphytes had not been carried out. Therefore, the following article is an approach to know the role of the Oak trees as well as their epiphytes that they hold in the storing process of C; this with the purpose of showing one of the many environmental services that this ecosystem provides.
Materials and Methods 
Study Area
The project was carried out in the Macanal Reserve located in the eastern Colombian Andean Mountain, in a town called Bojaca, located 27km far from Bogota. The study area is a montane cloud forest which is in a precipitous area with steep slopes, and it is located at 2700m above the sea level. The vegetation presents different levels of human intervention, and this is shown in the fragments of mature forests where mature Oak trees (Q. humboldtii) are the predominant species.
The climatology data of the area is registered by the Acapulco weather station of the "Instituto de Hidrología, Meteorología y Estudios Ambientales (IDEAM)". According to the 13 year-old data analysis (from 1990 to 2002), it has a precipitation with a bimodal behavior with an annual average of 61.5mm of rainfall; in which the most rainfall months that are March, April, and May in the first term of the year, and October and November in the second term. The average annual temperature is about 13º C, with the highest temperature on May with about 13.4º C and the minimum on July with about 12.6º C. The relative annual humidity average is about 91.9% for April, June, and July present the highest percentage with about 93%.
Methods
This study was carried out in an oak forest where
neither wood mining, nor any other kind of forest profit have taken
place. The methods used for measuring the tree volume were not
destructive at all. In fact, in order to measure the trees and the
epiphytes, five platforms were built over the upper surface of the
four Canopy trees - Q. humboldtii- to an average height of
20m and 23m. By means of a simple rope technique and tree-climbing
equipment, it was easy to have access to different parts of the four
chosen trees such the trunk and the crown as well as to the
platforms.
The approximate biomass of each evaluated tree was calculated from the tree volume and the wood density. For each tree, the trunk and the branches volume were calculated. For this, the length and the diameter of each trunk and branch were measured, and after that, we calculated the volume with the cylinder equation. In addition, the wood density was obtained from fragments of trunks and branches. For these samples, it was taken advantage of the natural falling of canopy oaks that were near the study area.
The biomass found in green leaves and upper branches of the crown was calculated from the gathering of six branches of different trees with approached dimensions of: 2m x 2m x 2m. All the leaves were taken off these branches in order to be dried and weighed. Subsequently, the number of branches that showed a covering of 2m x 2m x 2m was counted in each tree. With this, it was made an approximation of the number of branches and the quantity of biomass that these held.
The total biomass of bromeliads and organic matter placed on the bromeliads was evaluated in the selected trees taking into account the following parameters: 1) species, 2) number of individuals from each species in each host tree, 3) age class of the epiphytes. This was established from the gathering of 115 individuals that came in different sizes and the seven species that lived in the place. The diameter and the height were measured from these individuals, and then, leaves were taken off. Once the plant was stripped off, the bromeliad leaves were separated from the organic matter placed on the plant. Both samples were dried to a temperature of 70º C up to the point of obtaining a homogeneous drying to be weighted to obtain the biomass quantity after all. The analysis of bromeliads was carried out by visual estimation after having established the size.
Likewise, the biomass of lichens and bryophytes held in the crown tree was calculated from the selection of 32 branches of well-known volume with distinct diameters and located in different points of the crown of different trees. The lichens and bryophytes biomass held in the trunk was evaluated from six trunk parts of mature trees. All lichens and bryophytes were taken off to the 38 branches and trunk fragments, so that they could be dried to a temperature of 70º C with the purpose of obtaining the biomass value. The total biomass of lichens and bryophytes was calculated starting from generalizating about the samples of the whole tree volume.
The capacity of carbon sequestration in each component was determined in the laboratory by means of oxidation by using a dicromato potassium solution mixed with sulphuric acid measured colorimetrically. To sum up, 36 tests were made that were distributed in the following way: six samples of wood taken from the trunk, six samples of branches, six samples of green leaves, six samples of dead leaves, six samples of non-vascular epiphytes which included lichens and bryophytes, and, six samples of bromeliads. The sample C content was carried out at the "Corpoica - Tibaitata" soils laboratory.
Results 
The aerial biomass
of oak trees and their epiphytes
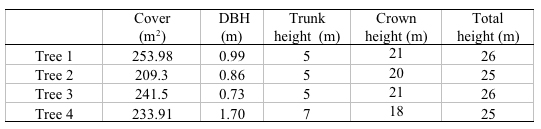
The evaluated aerial biomass was made up by the trunks, branches and leaves of each tree studied besides all the group of epiphytes that they held. The selected trees presented an average height of 25m and 26m, coverings between 209m2 and 255m2, and diameters between 0.86m and 1.70m (Table 1). The biggest percentage of biomass was found in the wood contained in the trunk, and the branches with a diameter higher than 5cm, where values fluctuated between 15 000kg and 25 000kg (Table 2). The branches with a diameter lower than 5cm were the ones that less biomass presented with an average between 58% kg and 78kg. Finally, it was found that the biomass of the green leaves had an average between 20kg and 28kg (Table 3).
In the case of the epiphytes associated to the oak tree and to the organic matter placed on the bromeliads, it was found that the biggest biomass was provided by the lichens and bryophytes with values between 36kg and 63kg, continued by the bromeliads whose biomass fluctuated between 6kg and 39kg, and finally, the organic matter placed on the bromeliads with values between 4kg and 23kg, which was made up mostly by dead oak tree leaves (Table 2).
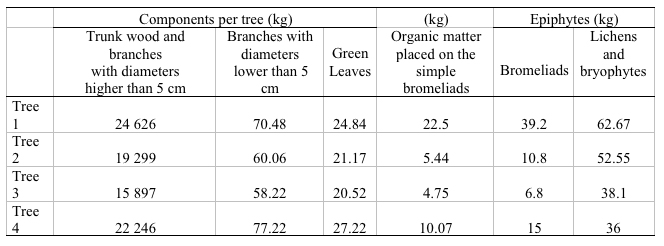
Table 2.Oak tree biomass distribution in each one of its components and the epiphytes distribution that grow in the crown
including the organic matter accumulated on it.
Sequestration and
Storage Capacity of C
The capacity of sequestration of carbon fluctuated in the different components of the oak tree. The values were between 35.95% and 40.13 % (Table 3). The component with the biggest capacity of C capture was that of the branches with diameters lower than 5cm with 40.13%, continued by the wood contained in the trunk and in the branches with superior diameters to 5cm with 38.75%; then, the fresh leaves with 35.95%, and finally, the dead leaves produced by the tree with 34.05% (Table 3)
On the other hand, in the epiphytes grown in the outer canopy it was found that the lichens and bryophytes presented 43% of C. These showed to be the component with the biggest C capacity of capturing inside the epiphytes epiphyte-host tree system. Bromeliads captured 38.82% showing a higher capacity of capture than of the components of their host tree, except by the superior branches. (Table 3)
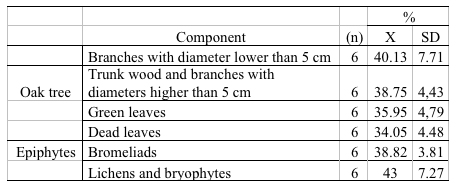
Table 3. C (%) capture capacity in oak trees (Q. humboldtii) as well as the epiphytes held on them. n = number of
individuals; X = average value; SD = standard deviation.
As for the C stored in the biomass it was found that in the four evaluated trees and their epiphytes, there was 32 066kg in 0.0938ha - corresponding to the sum of the coverings of the four evaluated trees -. The biggest quantity of C was found in the trunk and the branches higher than 5cm with values between 6 161 and 9 543kg. On the other hand, the branches lower than 5cm captured between 23.36kg and 30.99kg. Finally, the green leaves captured between 7.38kg and 9.78kg. Concerning the epiphytes, the lichens, and the bryophytes, the values were between 15.65kg and 26.95kg. The bromeliads had between 2.6kg and 15.2kg while dead leaves had between 1.62kg and 7.66kg (Table 4).
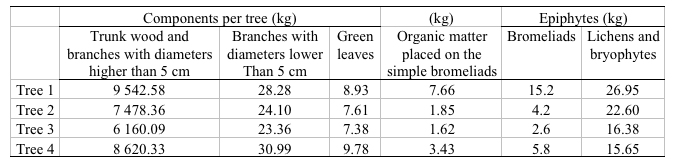
Table 4. Carbon content of tree stock in each component of the oak tree, their associated epiphytes and the intercepted organic matter.
Discussion 
One of the main factors that is affecting the montane cloud forests is Global warming which is directly associated to the growing presence in the atmosphere of greenhouse gases and the destructive emissions of the ozone layer (Hamilton 2001). In addition, the deforestation processes have reduced the original montane woods coverage in South America, and in fact, they are considered along with the tropical dry woods, to be the most threatened ecosystems in the world (Cavalier et al. 2001). Concerning oak forests it has been estimated that it is necessary at least a period of 65 years to restore the structure and floral composition, without taking into account epiphytes, and at least 84 years to reach the structure of a mature oak forest (Kapelle 2001).
Some years ago, before knowing the importance of oak forest fragments as protectors of basins of great importance for the hydric system, regulators of the regional weather conditions, and their role in the cleaning of air among other environmental services, the oak tree was used for carpentry purposes as well as agricultural purposes. It also was used for doing posts, fences, house and railroad beams, floors, bodywork, fine joinery, and coal, amongst other uses (UNESCO 2001). These uses without appropriate handling generated alterations in the storage of C in oak forests. Deforestation as well as the incorrect use of soil have produced a reduction in the flow of the C and in their percentages of storing. This change is due to the lower biomass of pastures and certain crops (Macera et al. 2000)
In the oaks evaluated, it was found that the percentage of C in the biomass of the host trees and their epiphytes was, in all the cases, inferior to 50% indicated as value for defect for the IPCC (1996), and inferior to values reported in other studies where it has been registered that the content of C of the wood of conifers is between 50 and 53% while in the species of wide leaf varies from 47 to 50% (Ramírez et al. 1997). These differences are probably due to that it is a mature forest where the efficiency of fixation of C is smaller, contrary to what happens in the secondary forests that are composed of species of quick growth (Denslow 1980), and that these can have a bigger efficiency in the fixation of the C that the primary forests (Ortiz 1997).
In spite of the low values in the concentration of C in the canopy oak trees and in their epiphytes, the epiphyte - host tree system presented high values of biomass which makes that this system can retain a significant quantity of C. However, it should be kept in mind that this study had as objective the estimation of the biomass of huge trees; therefore, the data that was obtained is generalized only to mature individuals of oak, and not to big extensions of vegetable covering, since in the forests there is not a continuity of this type of trees neither of its epiphytes. As a result, an overestimation of the aerial biomass can be made and, consequently, content of C can be mistaken. Brown & Lugo (1982) affirm that the presence of trees with big diameters can have a great influence on the vegetable biomass as well as the quantity of C.
To calculate the percentage of the sequestration and storage capacity of C in a forest has become a tool to keep big extensions of vegetable covering, since the storage of C helps to mitigate the global warming (Husch 2001). To be able to know in a more precise way which is the quantity of C that can store a natural forest it becomes necessary to calculate not only the wooden quantity in living vegetation, but also the biomass of all those herbaceous forms of life that grow in the soil as well as on the trees, since these forms of life are abundant in the tropical forests. In the case of the epiphytes, these are an important component of the tropical forests since they contribute significantly with the total of the biomass (Ingram & Nadkarni 1993), the diversity of species (Gentry & Dobson 1987), and in the cycle of several nutrients (Nadkarni 1984) of these ecosystems.
The forests of Quercus humboldtii have several important elements to take into account inside projects that imply forests like places that sequester and stock C, and conservation. These are forests of wide distribution along the three Colombian mountain ranges whose conserved relicts harbor big mature trees with a high biomass that are also covered by a great epiphytes biomass that at the same time intercepts a part of the organic matter that falls. All this biomass accumulated in the epiphyte - host tree system makes these forests to be potential resource to storing significant quantities of gases of effect greenhouse effect and in particular CO2; obtaining one more environmental service from these ecosystems.
Acknowledgements 
This project was carried out thanks to the economic support of the English organization Rufford, the support of field equipments Idea Wild and to the logistical support of Fernando Cortes, owner of the the Macanal reserve. We thank Sentido Natural Corporation (SN) for the given support through the project. Especially, we thank Néstor García, Héctor Gasca, Yolima Perez, Luisa Alvarez and Camilo Higuera for the manuscript revision. To Juan Carlos de Las Casas, and all the people who participated in the program of volunteers of SN for their company to field.
References 
Brown, S., C. Hall, W. Knabe, J. Raich, M. Trexler & P. Woormer. 1993. Tropical forest: their past present and potential future role in the terrestrial carbon budget. Water, Air and Soil. Pollution 70, 71-94
Brown, S. & A.E. Lugo. 1982. Storage and production of organic matter in tropical forest and their role in the global carbon cycle. Biotropica 14, 161-187.
Cavelier, J., D. Lizcaíno & M.T. Pulido. 2001. Colombia. In: Kapelle, M. & A. Brown. (Eds). Bosques nublados del neotropico.
Denslow, J. 1980. Gap partitioning among tropical rain forest tress. Biotropica 12 (2), 47-55
Dixon, R.K., S. Brown, R.A. Hougton, A.M. Solomon, M.C. Trexler & J. Wisniewski,. 1994 Carbon pools and flux of global forest ecosystems. Science 263, 185-190.
Doumenge, C., D. Gilmour, M. Ruiz -Pérez & J. Blockhus. 1995. Tropical montane cloud forest: conservation status and management issues. In: Hamilton, L.S., J.O. Juvik, & F.N. Scatena (Eds). Tropical montane cloud forest. New York, springer - Verlag p. 24- 37.
Gentry, A. & C. Dodson, 1987. Diversity and biogeography of neotropical vascular epiphytes. Ann. Missouri Bot. Gard. 74(2), 205-233.
Hamilton, 2001. Una campaña por los bosques nublados: Ecosistemas únicos y valiosos en peligro. In: Kapelle M. & A. Brown (Eds.). Bosques nublados del neotropico.
Husch, B. 2001. Estimación de contenido de carbono de los bosques. Valdivia pp. 87 - 91
Ingranm, S. & Nadkarni, N. 1993. Composition and distribution of epiphytic organic matter in a eotropical cloud forest, Costa Rica. Biotropica 25(4), 370-383
IPCC.1996. Intergovernmental Panel on Climate Change. Report of the twelfth session of the intergovernmental panel on climate change. Reference manual and work book of the IPCC 1996 revised guidelines for national greenhouse gas inventories. Mexico city, 11-12 September 1996.
Kapelle, M. 2001. Los bosques nublados de Costa Rica. In: Kapelle, M. & A. Brown (Eds.). Bosques nublados del neotropico.
Labastille, A. & J. Pool. 1978. On the need for a system of cloud forest parks in middle America and the Caribbean. Enviromental Conservation 5(3), 183 -190.
Lozano, G. & J. H Torres. 1974. Aspectos generales sobre la distribución, sistemática Fitosociológica y clasificación ecológica.
Masera, O., B.de Jong B & I. Ricalde. 2000. Consolidación de la oficina mexicana para la mitigación de gases de efecto invernadero. Sector forestal. Instituto de ecología UNAM & ECOSUR. 1-197
Nadkarni, N. M. 1984. Epiphytes biomass and nutrient capital a neotropical elfin forest. Biotropica 16(4), 249-256
Ortiz, R. 1997. Costa Rican Secondary forest: an economic option for joint implementation initiatives to reduce atmospheric C02. Draft paper presented for inclusion in the Beijer Seminer in Punta Leona. Costa Rica 19 p.
Ramirez, O, M. Gómez & S. Shultz. 1997. Valuging the contribution of plantation forestry to the national accounts of Costa Rica form the ecological economics perspective. Beijer Research Seminer. Costa Rica, 28 p.
Terborgh, J. 1985. The vertical component of plant species diversity in temperate and tropical forest. American Naturalist 126, 760-776.
UNESCO. 1981. Vegetation map of South America. Explanatory notes. Paris, UNESCO.
UNESCO. 2001. Tiempo decisivo para las selvas de neblina. Asuntos y problemas relacionados con el agua en los trópicos y otra regiones calido-húmedas. IHP Programa Trópicos Húmedos Serie No. 13. pp 37.
Vitousek, P. M., L.L. Loope & C.P. Stone. 1987. Introduced species in Hawaii: biological effects and opportunities for ecological research. Trends in Ecology and Evolution 2, 224-227.